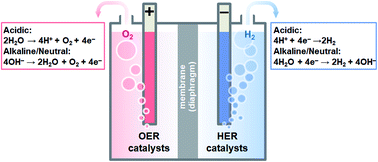
For the alkaline electrolysis cell water splitting is performed under alkaline condition. Generally dissociation of water to form H and OH intermediates is the key process for alkaline water splitting.

Reverse Electrodialysis Assisted Solar Water Splitting Scientific Reports
1

Noble Metal Free Electrocatalytic Materials For Water Splitting In Alkaline Electrolyte Sciencedirect
Low-cost alkaline water electrolysis has been considered a sustainable approach to producing hydrogen using renewable energy inputs but preventing hydrogenoxygen mixing and efficiently using the.

Alkaline water splitting. A pH of spot on 7 denotes a neutral solution neither acidic or alkaline. This reaction takes place in a unit called an electrolyzer. Water molecules have the chemical formula H 2 O.
In comparison with cells using acidic media water splitting in alkaline media broadens the selection of the electrocatalysts to non-noble metals or metal oxides. Alkaline water electrolysis is known as the principal process for the water splitting reaction. Environmental Science Cover Art.
Here we report a one-step approach to grow highly porous S-doped NiFe oxyhydroxide catalyst Energy. Alkaline electrolysis AEL AEL works with a liquid electrolyte in the form of potassium hydroxide. Seh et al Science 2017 The hydrogen evolution reaction HER 2 H 2 e H 2 is the cathodic reaction in electrochemical water splittingThe HER is a classic example of a two-electron transfer reaction with one catalytic intermediate and offers the potential to produce H 2 a critical chemical reagent and fuel.
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas by a process called electrolysisHydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Developing energy- and time-saving methods to synthesize active and stable oxygen evolving catalysts is of great significance to hydrogen production from water electrolysis which however remains a grand challenge. Efficient generation of hydrogen from water-splitting is an underpinning chemistry to realize the hydrogen economy.
The meaning of hydrolysis is a chemical process of decomposition involving the splitting of a bond and the addition of the hydrogen cation and the hydroxide anion of water. The adsorption configuration and adsorption energy E ads of H 2 O on different. The electrodes are made of metal.
However these molecules are capable of splitting up slightly in. Military applications related to the use of hydrogen isotopes boosted the development of this technology. The term is used broadly for substitution elimination and solvation reactions in which water is the nucleophile.
Any pH below 7 is acidic whilst any pH above 7 is termed alkaline. The process of splitting water into hydrogen and oxygen with the help of electricity has been used since the beginning of 19 th century. Hydrolysis h aɪ ˈ d r ɒ l ɪ s ɪ s.
Electrolysis is the process of using electricity to split water into hydrogen and oxygen. Low cost transition metals such as nickel and iron-based oxideshydroxides have. Sometimes called water splitting electrolysis requires a minimum potential difference of.
The first plants for the electrolysis of D 2 O D 2 production were built in Norway. Firstly the adsorption and dissociation of H 2 O are determined. Driving the HER with renewable sources of energy.
Electrolysis is a promising option for carbon-free hydrogen production from renewable and nuclear resources. From Ancient Greek hydro- water and lysis to unbind is any chemical reaction in which a molecule of water breaks one or more chemical bonds. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed.
Electrocatalysis of the hydrogen evolution reaction HER is critical to the operation of water-alkali electrolyzers 16 in which hydrogen is the main product and chlor-alkali electrolyzers 5 6 in which it is a side productThese two technologies are highly energy-intensive and are known to account for 25 to 30 87600 to 92000 GWhyear of the total electrical energy consumption.
Recent Advances Of Nonprecious And Bifunctional Electrocatalysts For Overall Water Splitting Sustainable Energy Fuels Rsc Publishing
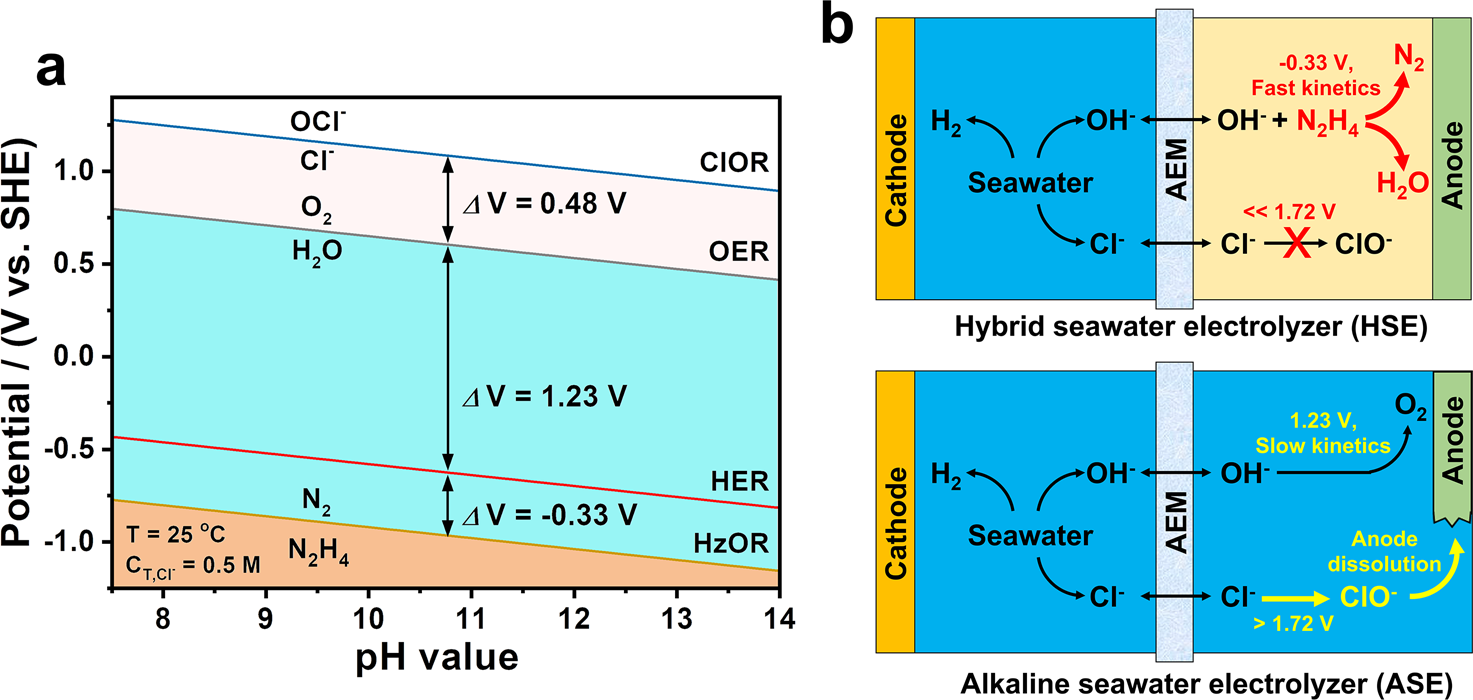
Energy Saving Hydrogen Production By Chlorine Free Hybrid Seawater Splitting Coupling Hydrazine Degradation Nature Communications
1

Overall Water Splitting A Schematic Illustration Of The Alkaline Download Scientific Diagram
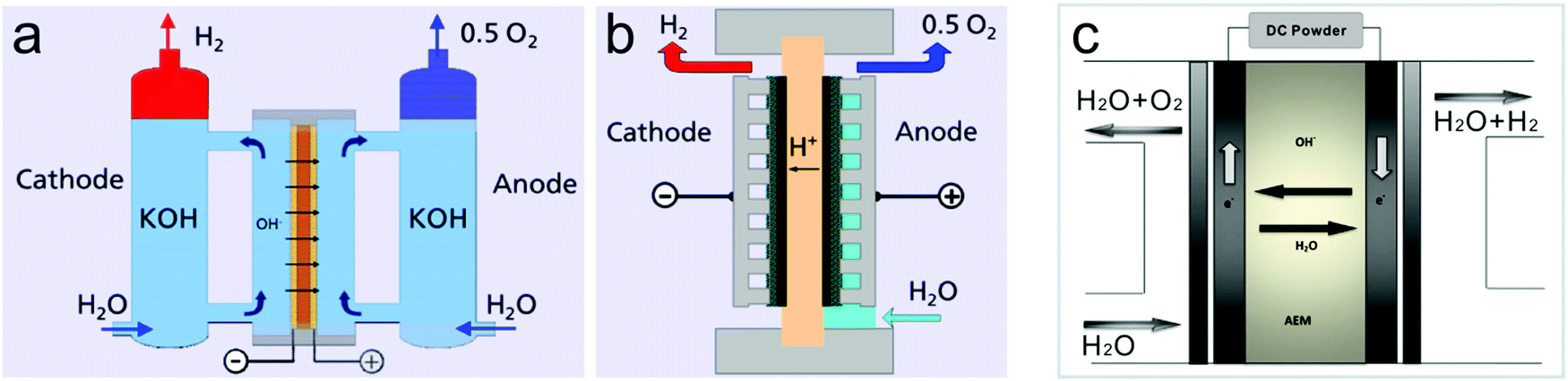
Recent Progress Made In The Mechanism Comprehension And Design Of Electrocatalysts For Alkaline Water Splitting Energy Environmental Science Rsc Publishing

Cobalt Stabilized Oxygen Vacancy Of V2o5 Nanosheet Arrays With Delocalized Valence Electron For Alkaline Water Splitting Sciencedirect

Nanosheets For Alkaline Water Splitting Chemviews Magazine Chemistryviews

Schematic Of Alkaline Water Electrolysis And The E Tac Water Splitting Download Scientific Diagram